Moments From Our Previous Sessions

ISO/IEC 17025:2017
This online training is designed to provide participants with a comprehensive understanding of ISO/IEC 17025:2017, the international standard for testing and calibration laboratories. It covers key requirements, including management system implementation, competence, impartiality, and consistent operations.
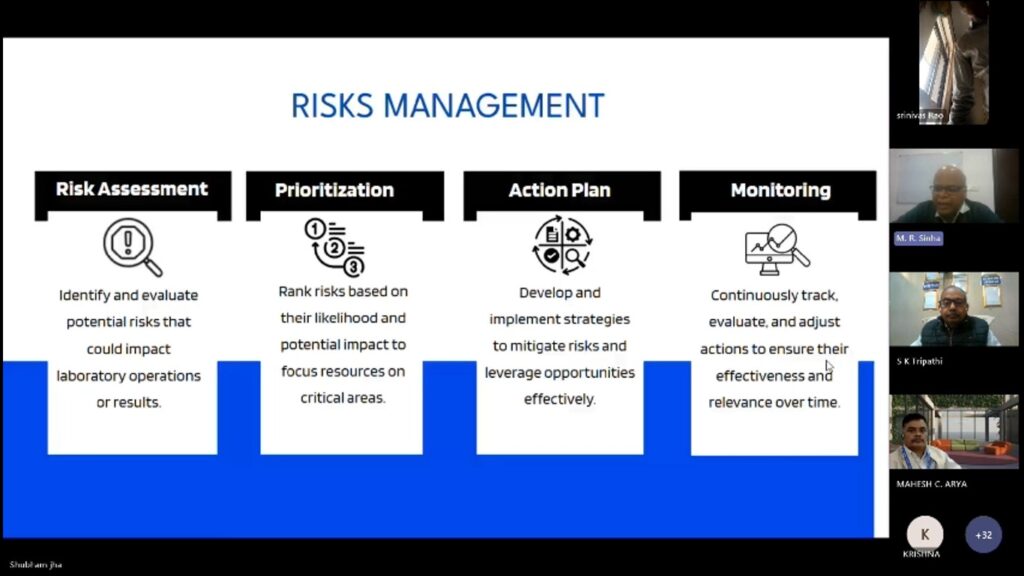
Participants will gain practical insights into quality control, internal audits, risk-based thinking, and accreditation processes.

Training Objectives
By the end of this training, participants will be able to:
✅ Understand the core principles and requirements of ISO/IEC 17025:2017.
✅ Gain in-depth knowledge of laboratory competence, impartiality, and management system implementation.
✅ Apply best practices for quality control, internal audits, and risk-based thinking.
✅ Ensure accurate, reliable, and consistent test and calibration results.
✅ Enhance laboratory efficiency, compliance, and accreditation readiness.
Who Should Attend This Training?
✅ Laboratory supervisors, quality managers, and professionals in calibration & testing.
✅ Individuals applying for or renewing ISO/IEC 17025 certification.
✅ Anyone seeking advanced knowledge of risk management in laboratory environments.
Training Format
Live Lectures
Expert-led discussions on ISO 17025
Handouts
Detailed reference materials for each topic.
Certification
Upon successful completion, participants will receive a training certificate.
Assessments
Short quizzes to evaluate learning progress.
Case Studies
Real-world case studies on ISO/IEC 17025:2017 implementation
Fees & Registration
💰 Discounted Fees: ₹4999 + GST (Original Price: ₹20000)
📅 Date: 6th -9th March 2025
🕒 Mode: (Online)
Why you should Enroll
This training is a valuable opportunity to gain industry-relevant knowledge from a professional with over 35 years of experience. Whether you are a laboratory manager, auditor, or compliance officer, this training will equip you with the tools to ensure accuracy, compliance, and operational excellence.